
If you do not wish to agree to these Terms of Use, do not access or use any part of the Web Site. By accessing or using the Web Site, you agree that you have read, understand and agree to be bound by these Terms of Use, as amended from time to time, as well as the Company Privacy Policy, which is hereby incorporated into these Terms of Use. Please read these Terms of Use carefully before accessing or using any part of the Web Site. All users of the Web Site are subject to the following website terms and conditions of use (these “Terms of Use”). If you are accessing the Web Site from outside the United States, Canada, or Puerto Rico, please see the appropriate international website, available at for applicable terms and conditions. These terms and conditions of use are applicable to the United States, Canada and Puerto Rico websites (“collectively the Web Site”) operated by VWR (the “Company”). HALO® Chromatography Columns and Consumables Sartorius laboratory instruments, consumables and services (TBC) PerkinElmer - Innovating for a Healthier World Quality Products from Sheldon ManufacturingĪgilent Chemistries and Supplies Portfolio Life Science Research Solutions, Products, and Resources VWR will support you from the latest life science products to the guaranteed purity of organic building blocks.
Retrieved 4 August 2022.A strong, vibrant research and development group is the lifeblood of all industries. Definition, Standards, and Procedures IUPAC Recommendation" (PDF).
National Institute of Standards and Technology. Thermogravimetry of potassium hydrogen phthalate and its use as a thermal standard. ^ "The Standardization Of NaOH and KHP Assay" (PDF)."Further Work on Potassium Hydrogen Phthalate as a Standard in Volumetric Analysis". ^ "104874 | Potassium hydrogen phthalate".For the latter, benzoquinone is suggested. Many TOC analysts suggest testing their instruments with two standards: one typically easy for the instrument to oxidize (KHP), and one more difficult to oxidize. Most TOC analyzers are based on the oxidation of organics to carbon dioxide and water, with subsequent quantitation of the carbon dioxide. KHP is also a useful standard for total organic carbon (TOC) testing. The pKa of KHP is 5.4, so its pH buffering range would be 4.4 to 6.4 however, due to the presence of the second acidic group that bears the potassium ion, the first pKa also contributes to the buffering range well below pH 4.0, which is why KHP is a good choice for use as a reference standard for pH 4.00. The buffering region is dependent upon the pKa, and is typically +/- 1.0 pH units of the pKa. KHP can be used as a buffering agent in combination with hydrochloric acid (HCl) or sodium hydroxide (NaOH). KHP dissociates completely in water, giving the potassium cation (K +) and hydrogen phthalate anion (HP − or Hphthalate −)Īnd then, acting as a weak acid, hydrogen phthalate reacts reversibly with water to give hydronium (H 3O +) and phthalate ions. It also serves as a thermal standard in thermogravimetric analysis. It is also used as a primary standard for calibrating pH meters because, besides the properties just mentioned, its pH in solution is very stable. KHP is slightly acidic, and it is often used as a primary standard for acid–base titrations because it is solid and air-stable, making it easy to weigh accurately.

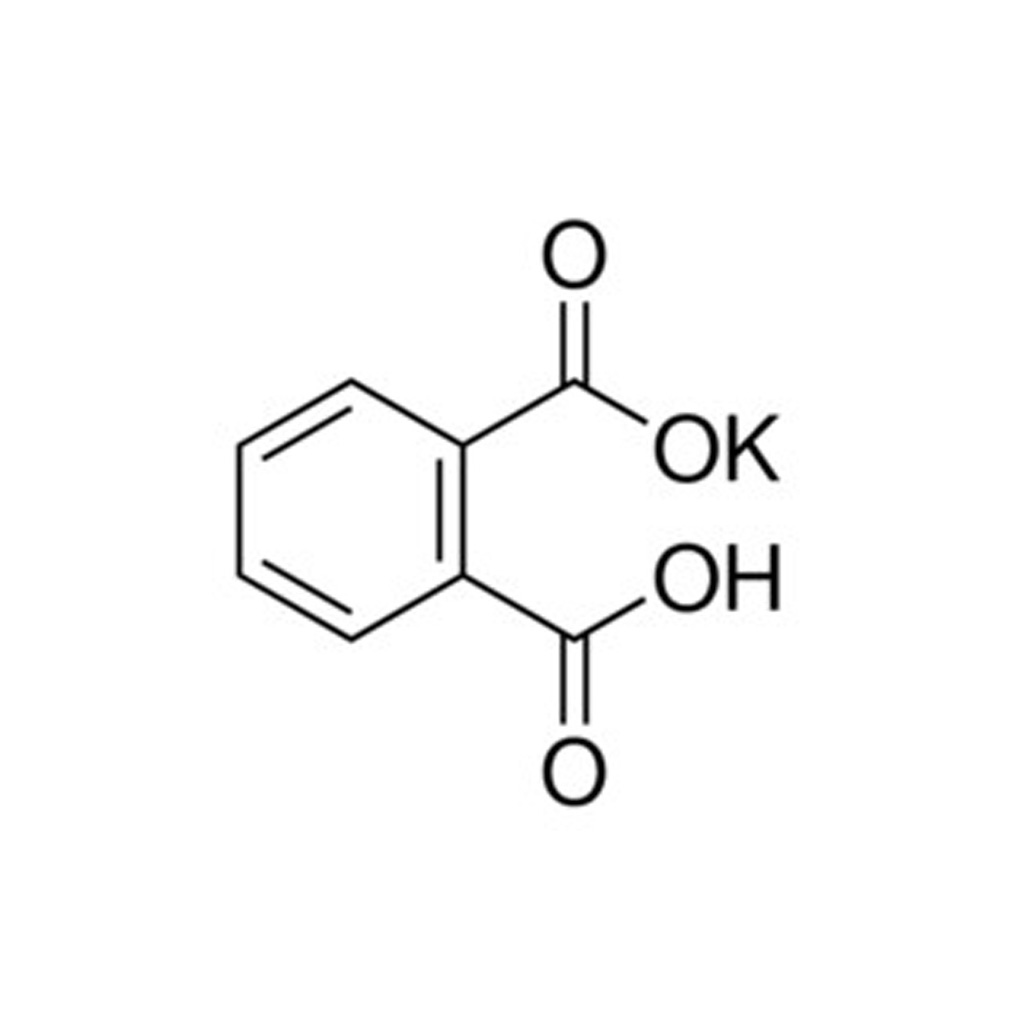
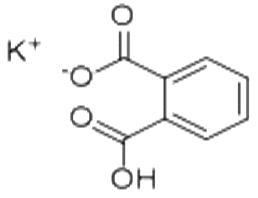
It forms white powder, colorless crystals, a colorless solution, and an ionic solid that is the monopotassium salt of phthalic acid. Potassium hydrogen phthalate, often called simply KHP, is an acidic salt compound.


 0 kommentar(er)
0 kommentar(er)
